DHM Dihydromyricetin
KINTAI'S Dihydromyricetin

Dihydromyricetin Introduction
Dihydromyricetin is also known as DHM, Ampelopsin. It is a naturally occurring flavonoid found in a variety of plants and has great medicinal value, used to treat fever, parasitic infections, liver disease and hangovers.
Natural DHM Manufacturers and Suppliers
The dihydromyricetin powder produced by Kintai is extracted from vine tea, with 98% dihydromyricetin, 30% water-soluble dihydromyricetin and 85% water-soluble dihydromyricetin. Our DHM powder has passed heavy metal, aflatoxin, pesticide residue and PAHS tests, providing customers with high-quality and safe products. It has a broad application prospect in the field of food, medicine and health care products.

KINTAI's dihydromyricetin specification
Other specifications can be customized according to your business needs


KINTAI's Dihydromyricetin Solubility
-KINTAI's 98% DHM powder, the maximum solubility is: 335ml water can dissolve 500mg DHM in 60-70℃. It has good solubility in hot water, almost completely dissolved at 75°C, and no crystals precipitate out when the temperature drops to room temperature.
-KINTAI's 30% water-soluble dihydromyricetin. The solubility results showed that 20g DHM could be dissolved in 500 ml of cold water.


Technical Data Sheet
| Product Name | Vine Tea Extract Powder | |
| Item No. | KT0922 | |
| Shelf life | 3 years when properly stored | |
| Technical Data | ||
| Plant information | Stem & Leaf of Ampelopsis grossedentata growing in China | |
| Method of Production | Solvent Extraction | |
| Solvent Used | Water & Ethanol | |
| Sterilization method | High Temperature & Presure | |
| Composition | 100% Ampelopsis grossedentata Stem & Leaf, no carrier, no chemicals or additives & No IRR/ETO treatment | |
| Packing | Typical 25kg inside or client's requirement; With foodgrade plastic innerbag, paper-drum outside |
|
| Storage | Storedin a cool and dry place, below 25℃, Humidity< 50%. Protectfrom moisture, strong light and heat. Sealedin its original package. | |
| Analysis Item | Specification | Method |
| Extract Ratio/Active Ingredient | ||
|
Dihydromyricetin (DHM) |
N.L.T. 98% |
HPLC |
|
Physical Items |
|
|
|
Appearance |
Off-white fine powder |
Organoleptic |
|
Taste and Odor |
Characteristic |
Organoleptic |
|
Cleanliness |
Absence of Foreign Matter |
Organoleptic |
|
Identification |
Positive |
TLC |
|
Sieve analysis |
100% through 80 Mesh |
USP34<786> |
|
Chemical Items |
|
|
|
Loss on Drying |
NMT 5.0% |
USP<731> |
|
Residue on ignition |
NMT 5.0% |
USP<281> |
|
Residual Solvent |
Meet requirement |
GC / USP<467> |
|
Residual Pesticides |
Meet requirement |
GC / USP<561> |
|
Heavy Metals |
NMT 10.0mg/kg |
CP2010 |
|
Arsenic (As) |
NMT1.0mg/kg |
ICP-MS |
|
Lead (Pb) |
NMT1.0mg/kg |
ICP-MS |
|
Cadmium (Cd) |
NMT1.0mg/kg |
ICP-MS |
|
Mercury (Hg) |
NMT 0.1mg/kg |
ICP-MS |
|
Microbiology Control |
|
|
|
Total Plate Count |
NMT 10,000cfu/g |
USP<2021> |
|
Total Yeast & Mold |
NMT 1000cfu/g |
USP<2021> |
|
E.Coli |
Negative |
USP<2022> |
|
Salmonella |
Negative |
USP<2022> |
|
Staphylococcus |
Negative |
USP<2022> |
|
Manufacturer certifies |
|
|
|
Food Grade |
Product is adapt for human consumption |
|
|
Preservative Used |
None |
|
|
Gluten |
Product does not contains gluten and is naturally gluten-free product, which compiles with COMMISSION IMPLEMENTING REGULATION(EU) No 828/2014 of 30 July 2014 on the requirements for the provision of information to consumers on the absence or reduced presence of glutenin food. |
|
|
GMO Status |
Product does not contain or consist of GMO's and is not produced from or does not contain ingredients produced from GMO's acc.To Regulations (EC) No.1829/2003 and 1830/2003. Product does not require GMO-labeling acc. To Regulations (EC) No.1829/2003 and 1830/2003. |
|
|
BSE/TSE Status |
Product is not derived from specific risk materials as defined in European Commission Decision 97/534/EC |
|
|
PAH |
Product is in accordance with Commission Regulation (EU) 2015/1933 |
|
|
Nanomaterials |
Product does not contain and is not in the form of engineered nanomaterials referred to Reg.(EC) 1169/2011 in point (t) of Article 2(2). |
|
|
Allergen |
Product is free of allergens according to Regulation (EC) No. 1169/2011 |
|
|
Doping Substances |
Product does not contain any doping substances listed on current WADA |
|
|
Pesticides |
Product is conform to the European Regulation, Directive 396/2005/CE and its modifications.
|
|
|
Vegan |
Product is suitable for vegan consumption |
|
|
Natural Origin |
Product is from Natural source |
|
|
Kosher |
Product is KOF-K Kosher certified |
|
Certificate of Analysis
| Basic information | |||
| Product Code | KT0922 | Product Name | Vine Tea Powder Extract |
| Botanical Source | Ampelopsis grossedentata | Plant Part Used | Stem & Leaf |
| Grade | Food Grade | Reference/Standard | In house requirement |
| Extraction Method | Solvent Extraction | Solvent Used | Ethanol & Water |
| Sterilization method | High Temperature & Pressure | Origin | China |
| Batch No. | 241017-KT0922 | Quantity | 200kg |
| Shelf life | 3 years when properly stored. | Manufacture Date | 17th,Oct 2024 |
| Composition | Stem & Leaf Of Vine Tea | Expired Date | 16th,Oct 2027 |
| Statement | |||
| Non-GMO/GMO Free | Yes | BSE/TSE Free | Yes |
| Allergen Free | Yes | Gluten Free | Yes |
| Non- IRR/ETO | Yes | Preservative Free | Yes |
| Nature Origin | Yes | Additive/Chemical | No |
| Kosher Certified | No | Halal Certified | Yes |
| Organic Certified | No | Vegetarian Suitable | Yes |
| Vegan Suitable | Yes | Other (Named) | N/A |
| Item | Specification | Result | Method/Reference |
| Physical Items | |||
|
Appearance |
Off-white Fine Powder Characteristic 100% through 80 Mesh Absence of Foreign Matter Positive |
Conformed Characteristic Conformed Not Detected Positive |
Visual Sensorial USP<786> Sensorial TLC |
| Chemical Items | |||
| Dihydromyricetin Loss on Drying Residual on ignition Residual Solvent Residual Pesticides Heavy Metals Arsenic (As) Lead (Pb) Cadmium (Cd) Mercury (Hg) |
98% Min 5% Max 5% Max Meet requirement Meet requirement 10ppm Max 2ppm Max 2ppm Max 1ppm Max 0.1ppm Max |
98.87% 1.09% 1.31% Meet requirement Meet requirement <10ppm <1ppm <1ppm ≤0.5ppm ≤0.05ppm |
HPLC USP<731> USP<281> GC / USP<467> GC /USP<561> CP2010 ICP-MS ICP-MS ICP-MS ICP-MS |
| Microbiology Control | |||
| Total Plate Count Total Yeast & Mold Escherchia Coli Salmonella species Staphylococcus aurreus |
10,000cfu/g 1000cfu/g Negative/10g Negative/10g Negative/10g |
Conformed Conformed Negative Negative Negative |
USP<2021> USP<2021> USP<2022> USP<2022> USP<2022> |
| The product could be used for human consumption. | |||
| Packaging: Packed in paper-drums and two plastic bags inside N.W.25Kg/Drum; G.W.28kg/Drum. Storage Store in a cool dry area, below25℃. Do not freeze. Keep away from strong direct light. |
|||
| Analysis | Zou Yuchi | QC Supervisor | Andy Chen |
Nutritional Profile of Vine Tea Powder extract
| Nutrient | Amounts |
| Energy(KJ/100g) | 1552 |
| Protein(g/100g) | 0.39 |
| Total Fat (g/100g) | 0.29 |
| Total Carbohydrate (g/100g) | 90.3 |
| Sodium (mg/100g) | <0.3 |
Analytical Procedures and Chromatogram
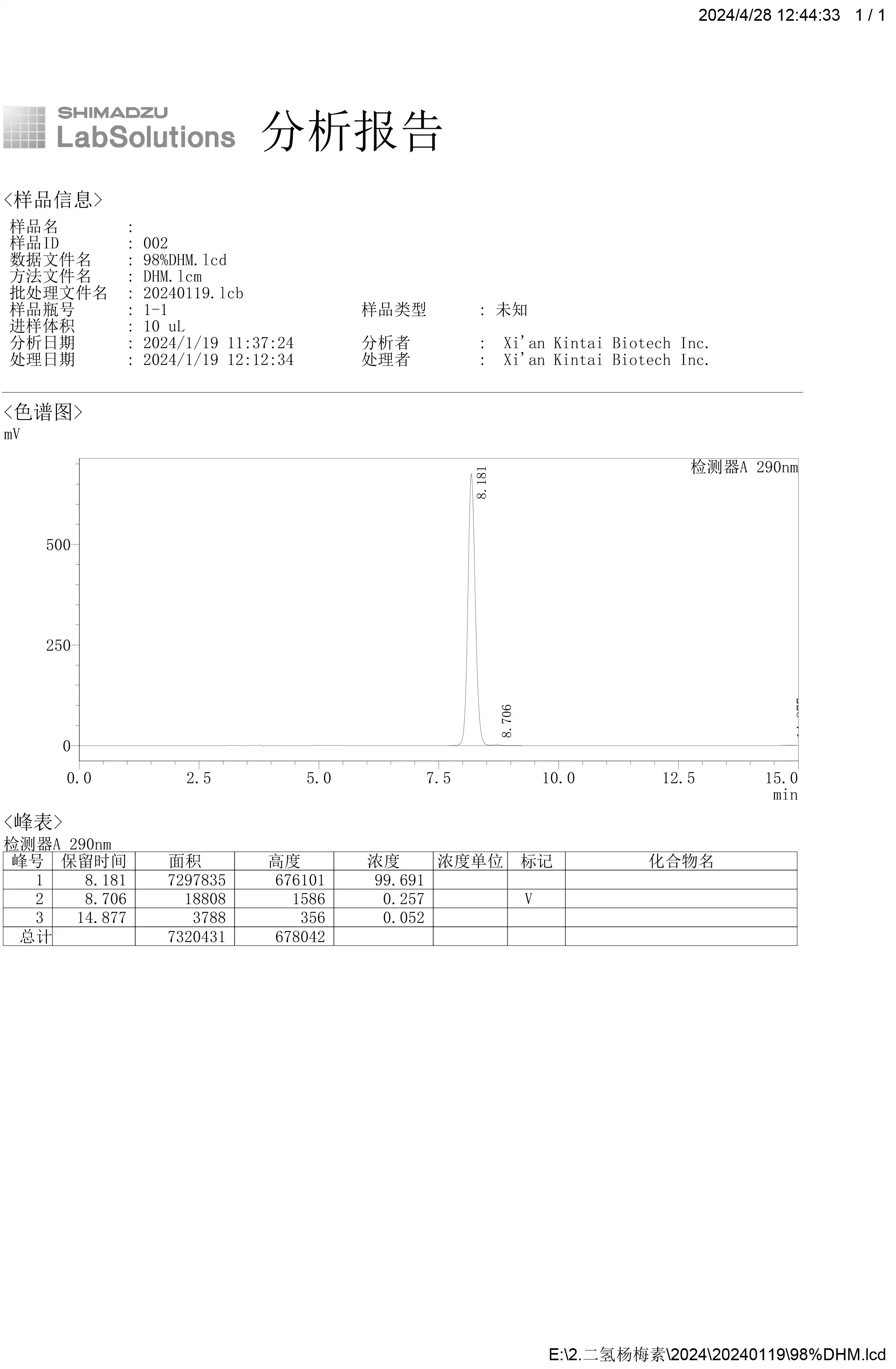
1. Instrument
A. SP880 HPLC
B. UV detector
2. Condition
Chromatographic Column ќ ROM-150 ODS-c18(4.6mm×250mm, 5ц)
Mobile Phase: Acetonitrile: 0.1% phosphoric acid water solution :Acetic acid (30 ∶70,V/V)
Flow Rate: 1.0mL/min
Wave Length: 294nm
Column: room temperature
3.Standardsolution
Transfer about 5 mg DHM standard (accurately weighed, to a 50mL volumetric flask, add the right amount Methanol to dissolve, Then add Methanol to scale mark, it is obtained.
4.Testingsolution
Transfer 5mg DHM powder, accurately weighed, to a 50ml volumetric flask, add the right amount Methanol to dissolve, Ultrasonic wave is shaken for 20 minutes till all dissolve in the Methanol. Place to room temperature Then add Methanol to scale mark, shake evenly, it is obtained.
5. Procedure
Separately inject equal volumes (about 20 uL) of the Standard preparation and the Assay preparation into the chromatograph, record the chromatograms, and measure the responses for the major peaks.
![]()
![]()
In which is A1 the assay sample , A2 is the standard peak area, W2 is the concentration of standard, W1is the concentration of assay sample.
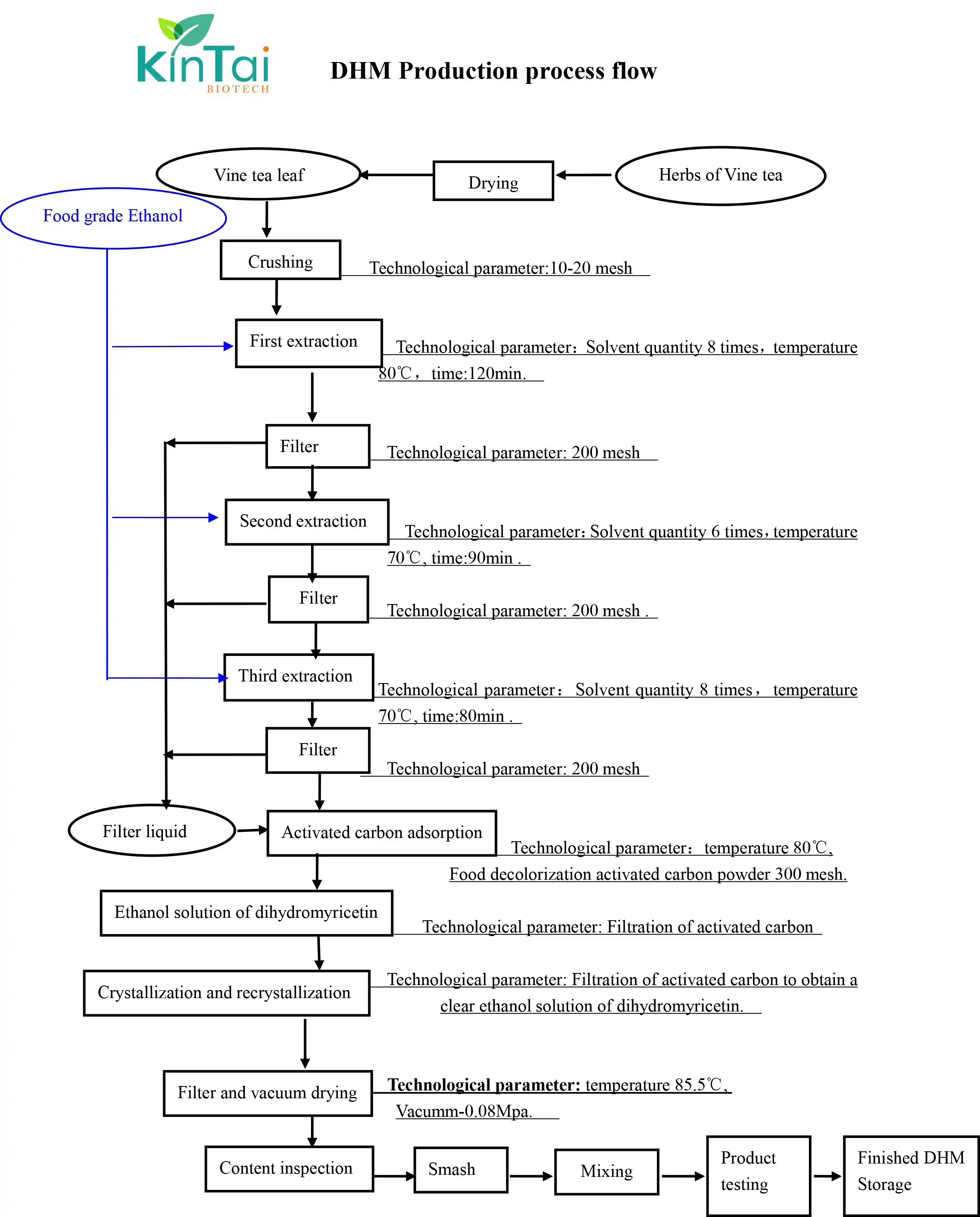
KINTAI's Dihydromyricetin extracion process
KINTAI's DHM Efficacy and its Application in the Marketplace






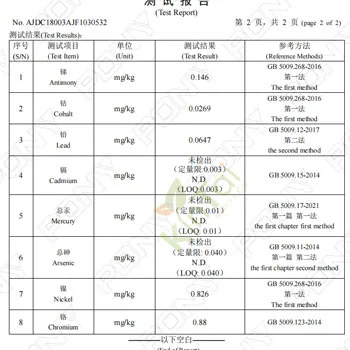
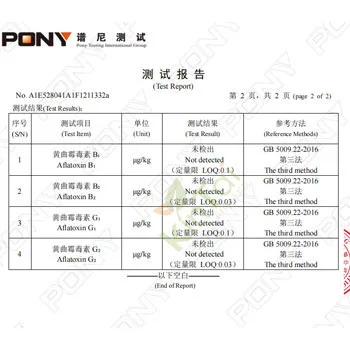
KINTAI's Certification




Contact us
As the world's leading manufacturer of vine tea extract dihydromyricetin powder, KINTAI has a standardized laboratory and a well-equipped R&D team. We strictly control product quality and can provide customers with high-quality products and absolute quality assurance. We support ODM and OEM services to provide customers with the best comprehensive services.
TEL: +86-181 8259 4708
Email: info@kintaibio.com
Office Address: West 16-3-1, Zhongnan Hi-tech Airport Industrial Port, Airport New City, Xixian New Area, Shaanxi, P.R.China.