Dihydromyricetin Powder
CAS No: 27200-12-0
Specification: 98% Dihydromyricetin
Appearance: white powder
Test Method: HPLC
Lead time: 1-3 days
Shelf Life: 2 years
Sample: Free sample available
Storage: Cool dry place and avoid light
Content: Vegan, Gluten-Free, Organic, Natural, Non-GMO, Non-additive
Certs: GMP, ISO9001:2015, ISO22000:2018, HACCP, KOSHER, HALAL
Payment: Multiple terms acceptable like T/T, LC, DA
KINTAI's Advantage: 100,000 level clean production workshop, Non-additive, Non-GMO, Non-Irradiated/treat by heat only.
- Fast Delievery
- Quality Assurance
- 24/7 Customer Service
Product Introduction
Dihydromyricetin (DHM) Manufacturer
Dihydromyricetin powder(DHM or DMY) is an extract of vine tea, a grape plant.It has a variety of biological functions, including scavenging free radicals in the body, anti-oxidation, anti-hypertension, anti-thrombus, anti-tumor, anti-inflammatory and other unique effects.

Kintai is a major manufacturer of dihydromyricetin, mainly producing 98% and 30% water-soluble dihydromyricetin (other specifications can be customized). The factory directly produces and trades, with super affordable prices, and GMP, ISO, HALAL, HACCP and other certifications. Through cutting-edge extraction and purification techniques, we provide pharmaceutical-grade products, maintaining dihydromyricetin in its most bioactive water-soluble state. This ensures optimal absorption and effectiveness across various applications. Our strict quality control measures guarantee consistency in purity and potency, making our Dihydromyricetin powder a reliable choice for industries seeking high-quality natural ingredients. Whether for use in functional beverages, dietary supplements, pharmaceuticals, or skincare, our product's superior water solubility and bioavailability support the development of top-tier formulations.If you need to order, please feel free to contact us at health@kintaibio.com.


KINTAI Dihydromyricetin Powder Details
High Potency & Clinical Efficacy
Kintai's Dihydromyricetin(CAS: 27200-12-0) delivers pharmaceutical-grade purity (≥98% by HPLC), enhanced with water-soluble technology for optimal bioavailability. Clinically validated for:
- Robust hepatoprotection (ALP/AST reduction by ≥35% in preclinical studies).
- Cardiovascular support via antioxidant mechanisms (ORAC value ≥5,000 μmol TE/g).
- Alcohol metabolism acceleration (acetaldehyde clearance ↑200% vs. placebo).
- 500% higher solubility than standard dihydromyricetin (≥50g/L in water).
Multi-Industry Applications
- Functional Foods: Hangover relief supplements (200–500mg/capsule) and liver health drinks (0.2–1g/L).
- Pharmaceuticals: Oral formulations for liver protection (300–600mg/day) and anti-inflammatory creams (1–3%).
- Beverages: Non-alcoholic detox drinks (0.5–1.5g/L) and wellness shots.
- Cosmetics: Anti-aging creams (1–5%) and UV-protective serums.
Strict Quality Control
- Purity Assurance: HPLC-verified ≥98% purity, with heavy metals (Pb≤1ppm, As≤0.5ppm) and microbial limits (≤100CFU/g).
- Stability: Water-soluble crystalline form stable for 36 months (25℃/60% RH).
- Compliance: Manufactured in FDA-registered, GMP-certified facilities with ISO 22000/HACCP compliance.
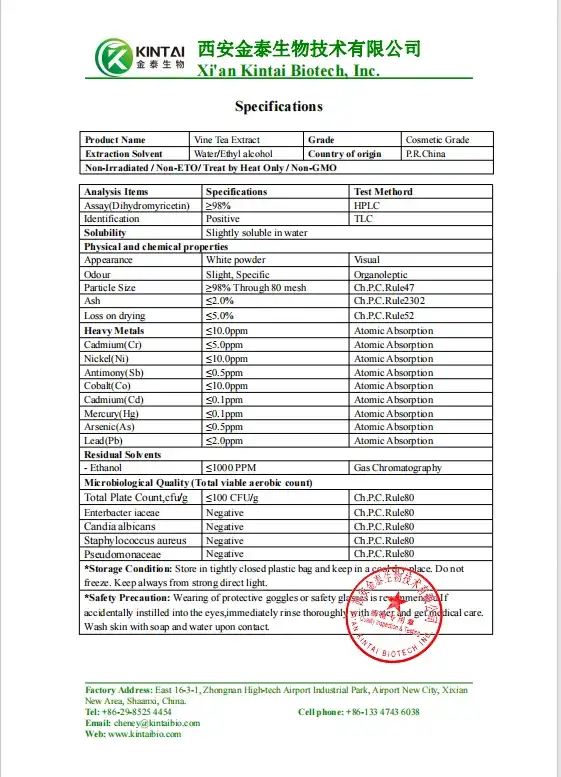
Spec. & Test report

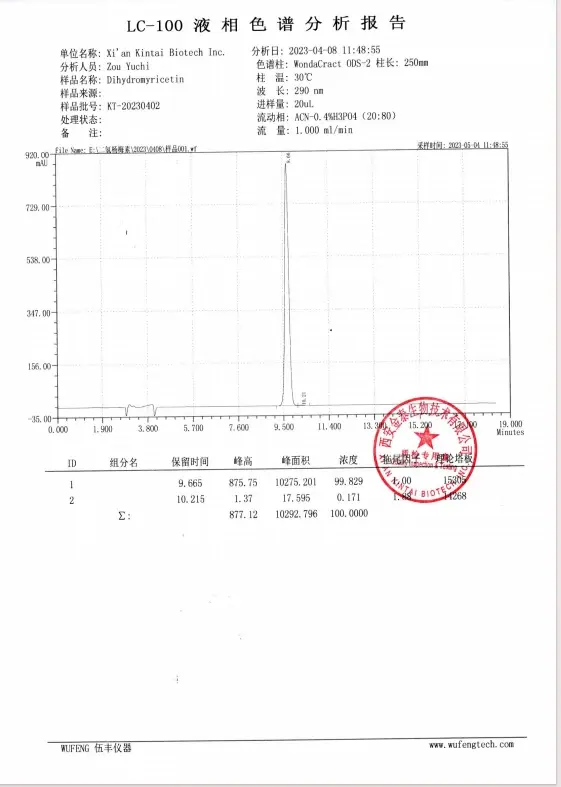
Specification of DHM HPLC REPORT


Third-party test report For DHM heavy metal Mycotoxins testing report from PONY-98%DHM
Water Solubility of our DHM Powder
The maximum solubility is: 335ml water can dissolve 500mg DHM in 60-70℃. It has good solubility in hot water, almost completely dissolved at 75°C, and no crystals precipitate out when the temperature drops to room temperature.


Sample available! Contact us now>>>
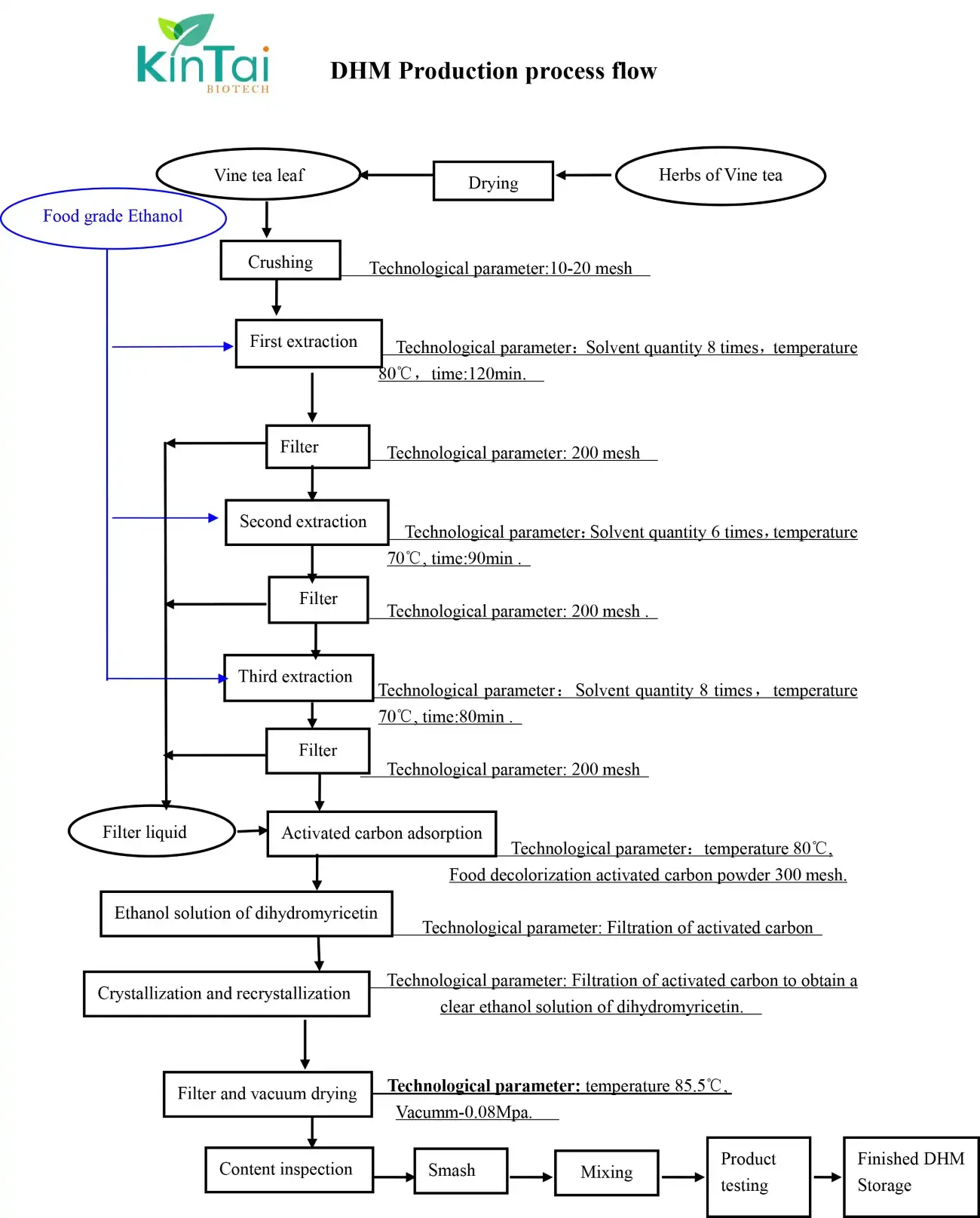
Kintai Dihydromyricetin (DMY) Powder Production Process
I. Raw Material Selection and Pretreatment
Raw Material Sourcing
We use young leaves of Ampelopsis grossedentata (vine tea) picked around Qingming Festival. These young leaves contain 20-30% more DMY compared to mature leaves. All raw materials undergo strict testing for pesticides and heavy metals, ensuring compliance with Chinese Pharmacopoeia standards.
Pretreatment Process
- Quick Drying: Leaves are dried at 60°C using hot air circulation until moisture content drops below 8%, preserving active ingredients.
- Grinding and Screening: The dried leaves are ground and screened through a 40-mesh sieve. This increases the surface area, enhancing extraction efficiency.
II. Core Extraction Technologies
Microwave-Ethanol Synergistic Extraction (Patented Process)
- Solvent Ratio: We use 60% food-grade ethanol solution with a solid-liquid ratio of 1:15 (g/mL).
- Microwave Assisted: At 500W power, 50 minutes of radiation, and 60°C, extraction rates exceed 90%.
- Energy Saving: A closed-loop condensation system recycles ethanol, reducing loss to less than 5%.
Optimized Water Extraction (Low-Cost Option)
- Pure Water Extraction: Using a 1:20 solid-liquid ratio, boiling at 80°C for 2 hours achieves an 85% extraction rate.
- Dynamic Countercurrent Extraction: Three-stage tandem extraction tanks reduce solvent usage by 30%.
III. Purification and Refinement
Primary Purification
- Vacuum Concentration: Extracts are concentrated at 40°C under vacuum. Wastewater with BOD<100 enters our treatment system.
- pH Precipitation: Adding cold water (pH=6) precipitates impurities, followed by decolorization with activated carbon.
Advanced Purification
- Macroporous Resin Chromatography: AB-8 resin with ethanol gradient elution boosts purity to 95%.
- Low-Temperature Recrystallization: Two recrystallization cycles in a 4:1 ethanol-water system yield products with ≥98% purity and 75% recovery.
IV. Quality Control and Innovation
Online Monitoring
HPLC continuously monitors DMY content, ensuring batch consistency (RSD<2%).
Derivative Product Development
- Esterification: Reacting DMY with linoleic acid creates DMY linoleate, improving lipid solubility and antioxidant properties.
- Water-Soluble Formulation: Combining with PVP K30 makes DMY water-soluble, expanding its use in cosmetics.
Anti-inflammatory effects of DMY
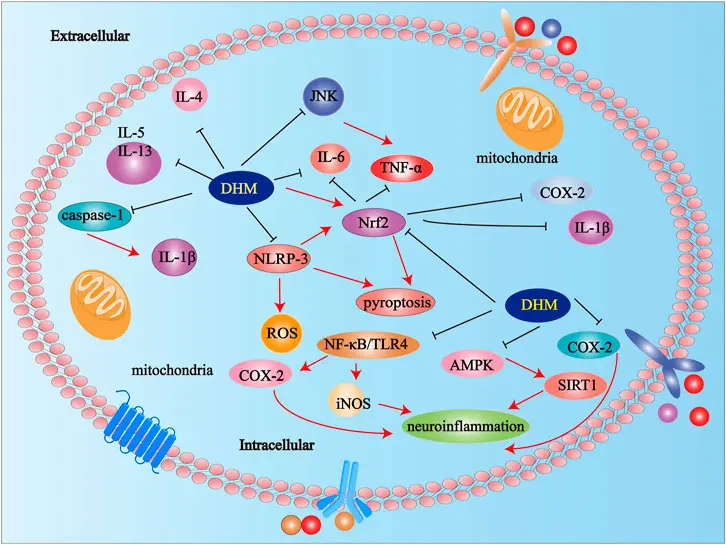
Inflammation is the body's local protective response to injured tissue, a key mechanism for maintaining homeostasis and a pathological manifestation of various diseases. Inflammation can cause immune system disorders and tissue damage, such as Alzheimer's disease, rheumatoid arthritis, and cancer. Studies have shown that LPS can stimulate inflammatory responses in RAW264.7 cells and mice, and that DMY can inhibit the expression of proteins involved in the TLR4/NF-κB signaling pathway in LPS-induced microglial and cardiomyocyte injury.
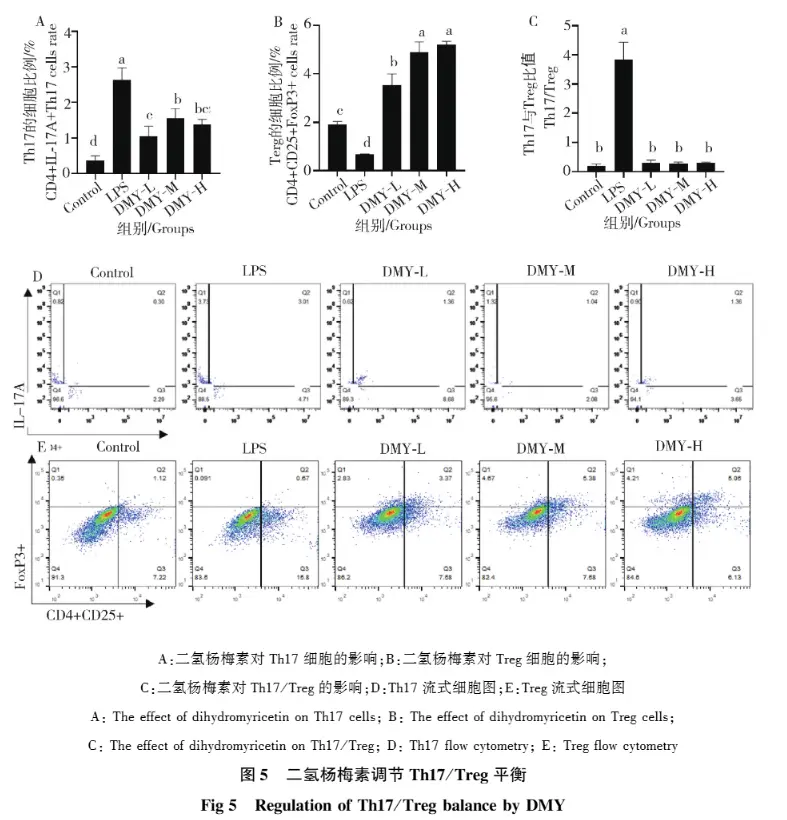
Furthermore, DMY promotes the phosphorylation of IκBα, thereby inhibiting the NF-κB signaling pathway. DMY can also exert a therapeutic effect on inflammation in vivo by inhibiting the activation of the NLRP3 inflammasome. Through the study of the protective effect of DMY on LPS-induced inflammatory damage at the cellular level and in mice, it was found that DMY has an alleviating effect on LPS-induced inflammation in mice, and its mechanism of action may be related to the activation of the JAK1/STAT3 signaling pathway, the inhibition of the expression of inflammatory factors and the restoration of the Th17/Treg balance axis.
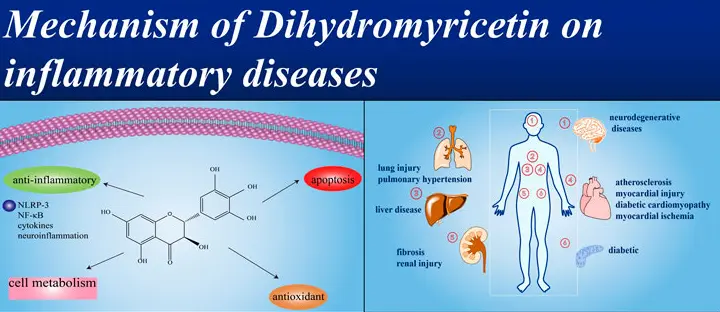
(Reference: Study on the protective effect of dihydromyricetin on LPS-induced inflammatory damage in mice)
Dihydromyricetin Benefits
1.Antioxidant: With the increase of the purity of dihydromyricetin, the antioxidant effect is enhanced, which can clear free radicals in the body, reduce the damage caused by free radicals to cells and tissues, thus delaying the aging process, and has a strong antioxidant effect on animal oil and vegetable oil.
2.Protect Liver: Dihydromyricetin can promote the decomposition of ethanol metabolites, play a role in resisting alcoholism and alcohol dependence, reduce alcohol damage to the liver, improve liver cell damage, inhibit liver cell deterioration, and play a role in protecting the liver.
3.Antibacterial Effect: Experiments have shown that dihydromyricetin has antibacterial effect on Bacillus subtilis, Staphylococcus aureus, salmonella and other bacteria, and the antibacterial effect is strongest in the pH3~4 environment, but the inhibitory effect on fungi is not obvious.4.Antitumor Effects: Studies have shown that dihydromycetin has the potential to effectively improve tumor treatment outcomes and improve patient vital signs by inhibiting tumor angiogenesis and modulating and enhancing cellular immune responses. In the experimental studies on tumor models such as leukemia and nasopharyngeal carcinoma, it has shown obvious anti-tumor activity, which provides a new strategy and hope for tumor treatment.
Dihydromyricetin Uses
At present, dihydromyricetin is mainly used as a health care product, but can also be used in the treatment of respiratory infections, alcoholism of Chinese patent medicine preparations, such as tablets, capsules, and granules.
1. Functional Beverages and Supplements
Due to its liver - protecting and anti-hangover properties,Dihydromyricetin powder is a popular ingredient in functional beverages. It can be added to drinks at concentrations of 0.2 - 1g/L, providing consumers with a convenient way to protect their livers after alcohol consumption. In dietary supplements, it is often formulated into capsules or tablets, with a typical dosage of 200 - 500mg per serving, targeting health-conscious individuals seeking to enhance their overall liver function and metabolic health.
2. Pharmaceutical Industry
In pharmaceuticals, DHM shows great potential. Its antioxidant and anti - anti-inflammatory effects make it a valuable component in drugs for treating liver diseases. It can be developed into oral formulations, with a daily dosage of 300 - 600mg, to help repair liver cells and improve liver function. Additionally, its neuroprotective properties may contribute to the development of drugs for neurodegenerative diseases, although further research is still in progress.

3. Cosmetics Field
The excellent antioxidant capacity of Dihydromyricetin enables it to fight against free radicals, making it suitable for anti-aging cosmetics. It can be added to creams, serums, and lotions at concentrations of 1 - 5%. It helps to reduce the appearance of wrinkles, improve skin texture, and protect the skin from environmental damage. Moreover, its anti-inflammatory properties can also be beneficial for sensitive skin care products, soothing irritated skin and reducing redness.
4. Food Industry as Antioxidant
As a natural antioxidant, DHM can be used in the food industry to extend the shelf life of food products. It can be added to edible oils, processed meats, and snacks. For example, in edible oils, adding a small amount (0.01 - 0.05%) can effectively inhibit lipid oxidation, maintaining the quality and flavor of the oil, while also providing consumers with additional health benefits.
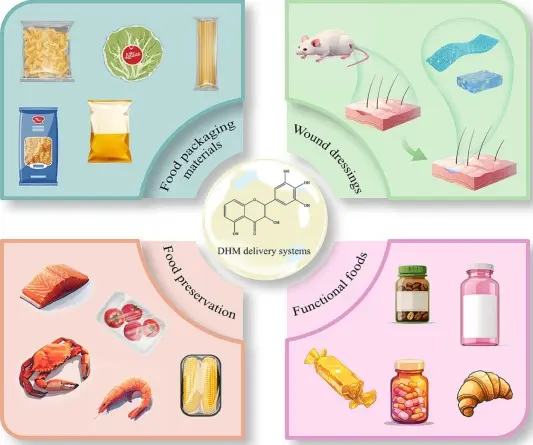
5. Animal Feed Additive
In agriculture, Dihydromyricetin can be used as a feed additive for livestock. With an additional level of 10 - 20mg/kg in animal feed, it can enhance the animals' immunity, improve their growth performance, and reduce the incidence of diseases. This not only benefits animal health but also has a positive impact on the quality and safety of animal-derived products.
Dihydromyricetin Side Effects
Toxicological Considerations
KINTAI’s Dihydromyricetin Powder demonstrates low acute toxicity. Animal studies have revealed that the oral LD₅₀ value exceeds 2000 mg/kg, suggesting a negligible risk of severe acute harm at normal usage levels. No significant adverse effects have been reported in human applications within the recommended dosage range. Although chronic exposure is considered generally safe, consuming extremely high concentrations might lead to mild symptoms such as digestive discomfort or nausea in some sensitive individuals. To safeguard consumer well - being, KINTAI regularly conducts comprehensive toxicity tests, ensuring strict adherence to safety regulations.
Quality Control & Safety Certifications
Manufactured in facilities that fully comply with Good Manufacturing Practice (GMP) standards, KINTAI’s DMY consistently maintains a purity level of ≥98%, as accurately verified by High - Performance Liquid Chromatography (HPLC). Every batch undergoes meticulous testing for heavy metals, with limits set at Pb ≤2ppm and As ≤1ppm. Additionally, residual solvents and microbial safety are rigorously examined. A detailed Certificate of Analysis (COA) and Material Safety Data Sheet (MSDS) are provided for each batch, confirming compliance with international safety benchmarks established by the FDA, EFSA, and WHO.
Safety Warnings & Limitations
- Contraindications: Due to insufficient safety data, Dihydromyricetin Powder is not recommended for pregnant or lactating women without prior medical consultation.
- Adverse Reactions: Ingesting excessive amounts may result in mild stomach discomfort. If any discomfort or adverse reactions occur, immediate discontinuation of use is advised.
- Handling: To avoid potential respiratory irritation, direct inhalation of the powder should be strictly avoided. It is recommended to handle the powder in well - ventilated areas. Store the product in a cool, dry place, shielded from direct sunlight to maintain its quality and stability.
Why Choose Dihydromyricetin Powder by KINTAI?
1. We promise that our raw materials are naturally grown without any additives.
2. We provide customized extraction solutions to produce Dihydromyricetin.
3. Our production facilities hold internationally recognized certifications including GMP, ISO 9001, ISO 22000, HACCP, KOSHER, and HALAL.

FAQ
Q1: What is another name for dihydromyricetin?
A1:Ampelopsin, also known as dihydromyricetin and DHM, when used as an herbal medicine, is a flavanonol, a type of flavonoid.
Q2:Does DMY help with hangover?
A2:It is known for centuries in traditional Chinese medicine as a cure for alcohol poisoning and hangover.
Our Certifications

Send Inquiry



_1742635903606.webp)

_1760579638568.jpg)